What is a decentralized clinical trial?
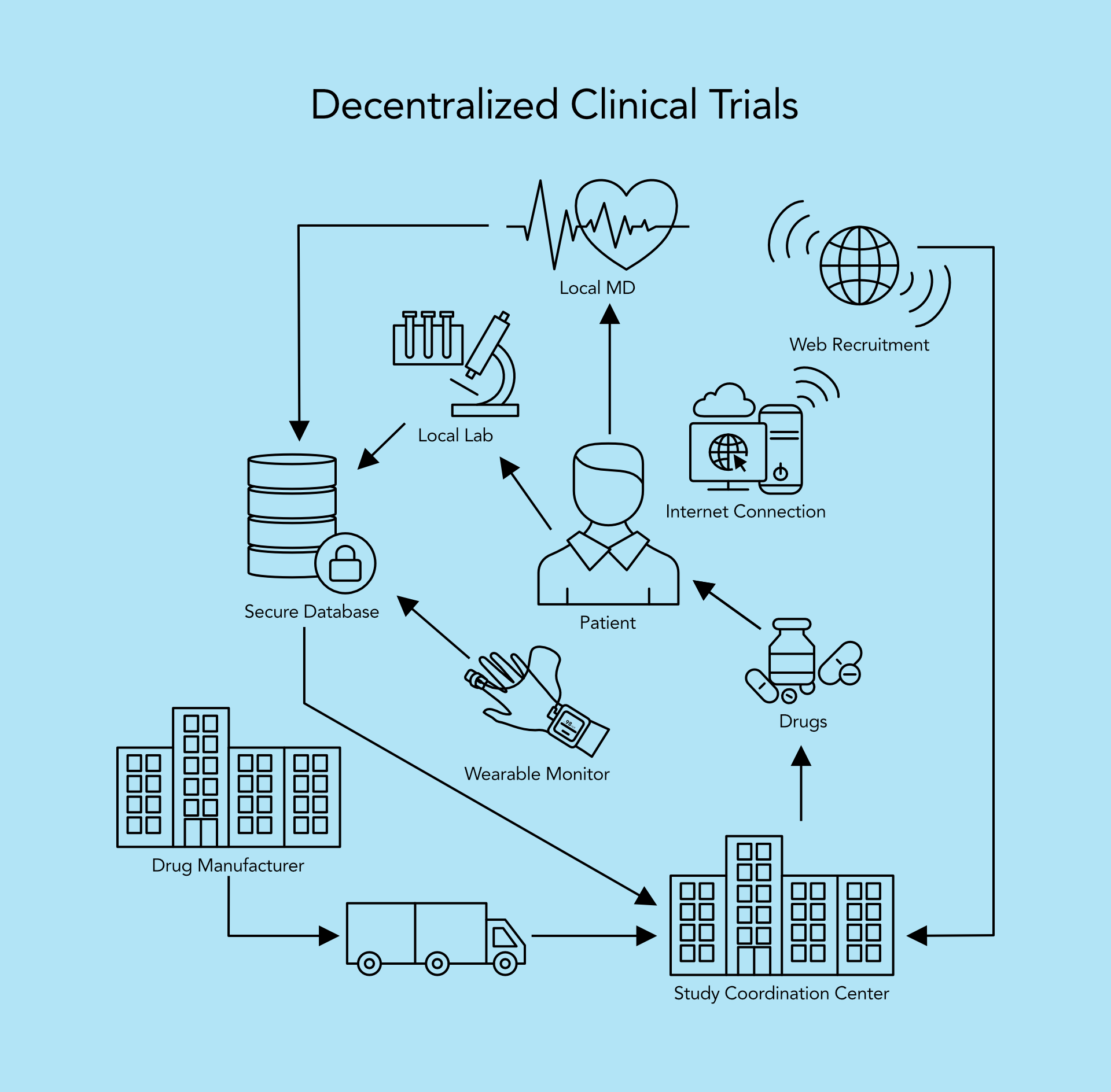
A decentralized clinical trial means that almost all the steps are performed without in-person contact between the team that is carrying the trial and the subject of the trial. Decentralized clinical trials are differentiated from traditional clinical trials by using various technologies that enable remote or virtual communication. This kind of trial can be also referred to as virtual, remote, direct-to-participant.
Since it is currently impossible to eliminate the need for in-person contact between study teams and study participants, the word “hybrid” is more appropriate for these kinds of studies.
Source: Van Norman, G.A. J Am Coll Cardiol Basic Trans Science. 2021;6:384-287.
Technologies used for decentralized clinical trials
The heart of a decentralized clinical trial is a web cloud-based platform, with the whole system relying on telehealth technology. Its subdomains – remote monitoring, wearable devices, electronic records (eConsent, eCOA) and other clinical tools are used for maximizing efficiency and safety.
Among the different tools used for Decentralized Clinical Trials, we will focus on remote monitoring as the most interesting parts from the technology side.
There are progressively more wearables that are growing out of the ordinary consumer grade and getting medically approved, and those wearables are generating digital endpoints that are replacing some of the traditional endpoints. Two devices from our production stand out when it comes to remote monitoring – Humeds Cardia and Marani CTG.
Humeds is a complete telehealth solution, which enables simple and comfortable ECG recording and analysis, combined with the quick, automatic and precise AI supported diagnosis of cardiac arrhythmias.
Marani’s innovative telehealth solution provides high-quality, clean, AI-processed data, which flows back to mobile phones in real time and provides physicians with key fetal and maternal parameters to better manage their patients in a remote setting. HTEC has developed hardware, firmware and software with the algorithms for fetal ECG and uterine activity detection.
Benefits of decentralized clinical trials
Here’s why decentralized trials are creating a better patient experience. They are:
Patient-centric
DCTs can be tailored to many different patients.
Scalable
A well-designed decentralized clinical trial with a solid software architecture is easily scalable to encompass many participants and gather a vast amount of data.
Mostly remote
Reduce unnecessary in– person contact. This removes or at least alleviates geographical restrictions – it allows access to a larger, more diverse population of participants leading to higher quality data. It also increases adherence and retention.
Digitalized
The information is collected and processed digitally, reducing redundance, error, and delay. The amount of data is increased in comparison to centralized clinical trials, collected at multiple points in time, or continuously. This is more precise than data collected only at a medical facility at one point in time. This data is available on a central platform, enabling analyses and gaining new insights.

Challenges for decentralization
As any novel thing in the healthcare and life sciences industry, decentralized clinical trials adoption has its challenges. The industry is highly regulated, and stakeholders are wary as a result.
Additionally, there are some challenges that are inherent to the remote or decentralized systems, as well as those related to the large throughput of data:
- Since most things can be done remotely, a study may involve participants who are unable to leave their homes. In this case, in-person contact is occasionally necessary. As a result, study team member must travel great distances to visit those participants
- Some participants have difficulties in understanding new technologies and prefer in person visits
- The supply chain issues that arise in cases that there is a need for a technology dependent on offshore pathways (i.e., chips manufacturing)
- Finding reliable vendors that can satisfy all the technical, legal and security standards. With its deep understanding of regulatory frameworks and long-term experience building transformative Health and Life Science solutions, HTEC Group brings all the pieces of the puzzle together helping clients reimagine clinical development and power patient-centric design.
- Organizing logistics for delivering equipment and supplies
- Preventing technical failures in the system that processes the data, sometimes resulting in the loss of parts of the data
- Regulations – Regulators have recognized the potential benefits of DCTs, and consequently, they have begun to issue guidance documents addressing the unique aspects of DCTs, such as data integrity, remote monitoring, electronic informed consent, and patient privacy. However, the absence of harmonized international regulations can pose challenges for sponsors and CROs seeking to conduct global DCTs, as they must navigate a complex landscape of varying regional requirements.
Regulatory compliance – a critical component of DCTs
For both CROs and sponsors, regulatory compliance is a vital part of decentralized clinical trials. Sticking to an ever-changing set of criteria and requirements can be time-consuming and difficult, especially when handling multi-center, worldwide trials. CROs and sponsors must ensure that their study designs, data-gathering methods, and patient engagement initiatives are in accordance with local laws.

Ethical challenges in decentralized clinical trials
Ethical difficulties in decentralized clinical trials deserve special consideration since they can influence many elements of trial conduct and outcomes.
From the technology point of view, these are the top five ethical challenges that must be addressed:
Informed Consent
Ensuring that participants completely understand a trial’s purpose, procedures, risks, and benefits is critical. Because of the remote nature of the process, obtaining informed consent in DCTs may be more difficult. Electronic informed consent solutions can be used to get and document consent, but it is critical to ensure that the consent process is clear, transparent, and accessible to participants with diverse levels of digital literacy.
Equity
While DCTs have the potential to expand patient access to clinical trials, there is a concern that they could exacerbate current healthcare disparities. Participants from low-income families, those living in remote areas, or those with inadequate computer literacy may face challenges to participation. Solutions that reduce these barriers and promote inclusion must be developed to provide equitable access, such as providing user-friendly interfaces, enabling remote help, and making trial materials available in many languages.
To learn more read “Are Digital Health Solutions Widening the Health Equity Gap?”.
Data Privacy and Security
Because sensitive health information is frequently exchanged and kept electronically, protecting patient data is a major ethical problem in DCTs. To preserve participant privacy and maintain trust in the research process, it is critical to implement strong data security measures and comply with data protection legislation such as GDPR and HIPAA.
Participant Burden
DCTs may need users to acquire and send data using various devices and technologies. It is critical to reduce participant burden and guarantee that the study does not have a detrimental influence on their quality of life. This can be accomplished by selecting user-friendly devices and applications, providing proper training, and providing ongoing assistance during the trial.
Risk-Benefit Analysis
Applying new technologies and remote monitoring can create additional dangers compared to ordinary clinical trials, such as equipment malfunction, data loss, or data misinterpretation. Researchers and sponsors must regularly assess the risk-benefit balance throughout the trial to meet ethical criteria.
Addressing these ethical difficulties in DCTs is critical for protecting participants and maintaining the research process’s integrity. Researchers may ensure that DCTs contribute positively to medical advancements while keeping the greatest patient safety and well-being standards by carefully considering these concerns and adding ethical considerations into trial design and implementation.
CROs and sponsors have vast experience in running clinical trials, but this new reality brings new challenges to the table. Regulations evolved mostly to follow the technology-enabled distributed nature of DCTs.
Authorities have recognized the potential benefits of DCTs. Consequently, they have started issuing guidance documents addressing the unique aspects of DCTs, such as data integrity, AI-driven data analysis, wearables and telemedicine, remote monitoring, electronic informed consent, and patient privacy. This constant catching-up game then impacts the DCT performance as new and evolving guidelines must be implemented. Moreover, the absence of harmonized international regulations poses challenges for sponsors seeking to conduct global DCTs, as they must navigate a diverse landscape of varying regional requirements.
Moving to more technology-supported clinical trials can be overwhelming for sponsors, CROs and patients. This is why having a trustworthy technology provider greatly impacts execution time, costs and trial accuracy.
How to transform the clinical trial paradigm and build a patient-centric ecosystem?
Although there are still challenges to the wider adoption of DCTs, it has become evident that this category of trials enables more patient-centric, efficient, and precise studies while providing a swath of new opportunities for acquiring knowledge based on high volumes of data they provide. As is the case with any new technology in medicine, only a sincere collaboration between all the relevant stakeholders will enable a full adoption.
At HTEC, we breathe technology and have years of experience in healthcare and life science. For sure, we can help optimize the procedures, maintain compliance, and ensure data integrity by providing specific software and digital solutions customized to the unique demands of DCTs. We are helping sponsors and CROs seamlessly integrate modern technologies and global regulatory requirements to boost trial efficiency.
About the authors
Nemanja Kovacev — A physician and software engineer with 15 years of combined medical and software development experience. With a PhD in orthopedics and digital health, this AI in Healthcare specialist is the lead author and a co-author of medical, engineering, and popular science papers. Nemanja participated in a number of national and international medical and IT conferences as a keynote speaker and as a session chair. Currently working as a MedTech Expert at HTEC Group, as well as a practicing Orthopedic and Trauma Surgeon, and Digital Health Advisor.
Milos Cigoj — As a compliance and quality lead at HTEC, Milos has an outstanding experience in helping evangelize the importance of quality and compliance in medical device software development. He joined HTEC to, as he explains, “help HealthTech ecosystems overcome hurdles ahead before starting to smooth the path for disruptive medical technologies and benefit patients globally.”
__
To learn more about how we can help you build transformative medical solutions and contribute to better care for all, talk to our experts or connect with Nemanja Kovacev or Milos Cigoj. In the meantime, check out our success stories to see how we help provide better health for all.





